To begin, let’s take a moment to define each term for clarity:
Quality Control (QC) is defined as an aggregate of activities designed to verify that clinical data are generated, collected, handled, analyzed, and reported according to protocol, Standard Operating Procedures (SOPs), and Good Clinical Practice (GCP) guidelines. In clinical research, this is used to ensure the protection of the patients and participants by the reduction of risk, reliability of data collected and assurance of consistent internal processes. QC focuses on the testing of products to meet established quality standards. Examples of QC include:
- Review and inquiry of the protocol by the Institutional Review Board (IRB), an ethics review committee designed to ensure the safety of the human subject within the clinical trial domain,
- Ensuring proper execution of the Informed Consent for all subjects enrolled at a site into the clinical trial.
- Select a random product, test it, and rank it on a chart. The testing parameters should be measurable and quantifiable. The chart should determine if the product meets the set quality standards or shows how much it varies if it does not.
- In manufacturing, this could be testing different stages of production, from raw material to the final output, to detect if dangerous microbiological elements occur could require the need to change packaging and storage conditions.
Quality Assurance (QA) is defined as a planned, systematic, and independent evaluation of trial ‐related activities and documents to verify data has been generated, collected, handled, analyzed, and reported according to protocol, SOPs, and GCPs. The goal is to minimize the risk of errors, bias, and fraud, to ensure trial results are trustworthy and help support clinical decision-making. Examples of QC include:
- Verification of data writing on source documents or in medical histories against what is entered in the electronic data capture database (EDC) of the sponsor.
- A sponsor hires a clinical trial monitor to go to a clinical site every month to review materials generated through a clinical trial that is enrolling.
- In the laboratory, regularly calibrate equipment and machines before starting experiments or tests.
QA and QC are both concerned with:
- Eliminating defects within a process or system
- Mitigating risk to provide safety for the environment and/or human
- They share the same goals as the stakeholders under the QMS umbrella
- They both ensure a product or service meets a certain standard of quality

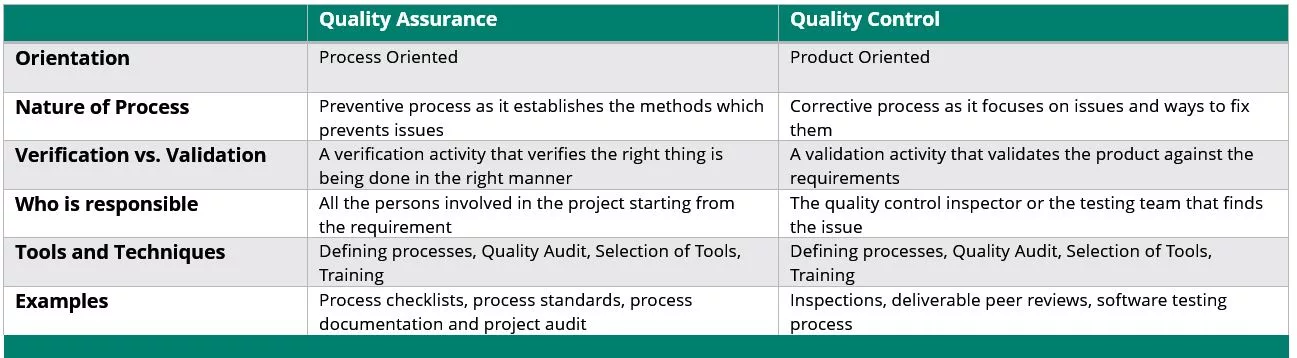
Simply put, within the sphere of GCP, QC is a subset of QA. QA is extensive and evaluates the whole process from making the product to its use, while QC is more focused in the inspection process to validate quality and identify problems or issues. QA shows the overall process from prevention to corrective action, whereas QC points out the problem.
- ICH E6(R2) GCP Guidelines have “Quality” Guidance we recognize:
- All clinical trial information should be recorded, handled, and stored in a way that allows its accurate reporting, interpretation, and verification (2.10)
- Systems with procedures that assure the quality of every aspect of the trial should be implemented (2.13)
- Quality control (QC) should be applied to each stage of data handling to ensure that all data are reliable and have been processed correctly (5.1.3)
- ISO 9001:2015 is another example of one of the most recognized and implemented quality management systems in the world.
- The United States Food and Drug Administration also defines “Quality” regulations and guidance under Investigator and Sponsor responsibilities
QC is focused on the inspection and testing of products or processes to meet established quality standards. It aims to identify issues, validate quality, and ensure reliable outcomes. Examples of QC activities include reviewing protocols, ensuring proper execution of informed consent, and conducting random product testing.
FDAQRC can help with any step of your Quality Management process. We have GCP experts on hand who value Quality standards and are experienced with Quality Assurance, Quality Control, and Quality Improvement.
Amy Fox is a full time Auditor for FDAQRC with over 20 years of experience in clinical trial operations and regulatory compliance. She holds her Certified Clinical Research Coordinator (CCRP) with ACRP. Her areas of expertise include GCP and early phase human pharmaceutical clinical research.


A Closer Look at Inspection Readiness: Mock/Hats On-Off Approach

How to Secure Project Assignments with FDAQRC: A Guide for Consultants

Tips For Preparing & Hosting a Successful Audit

Regulating AI in Clinical Trials: What ICH E6(R3) Does (and Doesn’t) Say

Explores the Differences Between Auditors and Inspectors in the Clinical Research Industry

Competency Based Programs Contribute to Early Clinical Research Professional’s Training

15 Years of Excellence: FDAQRC's Journey and Future Vision









