Business
Consultant Resources
Downloadables
Expertise
News & Events

Tips For Preparing & Hosting a Successful Audit
Turn audit stress into strategic success with these expert preparation and hosting tips

Regulating AI in Clinical Trials: What ICH E6(R3) Does (and Doesn’t) Say
Despite AI’s growing role in clinical trials, ICH E6(R3) remains silent—does this leave room for innovation or uncertainty?

Explores the Differences Between Auditors and Inspectors in the Clinical Research Industry
Despite similarities and differences in their roles, both auditors and inspectors play an important part in ensuring compliance.

Competency Based Programs Contribute to Early Clinical Research Professional’s Training
Explore the benefits of on-the-job training for Clinical Research Professionals

15 Years of Excellence: FDAQRC's Journey and Future Vision
Marking a milestone of 15 years, FDAQRC continues to grow with expanded global services, innovative partnerships, and steadfast commitment to quality and compliance.

Tips for a Positive Outcome While Performing a Challenging Audit
Transform a daunting audit into an opportunity for improvement with strategies to de-escalate tensions and foster collaboration

How To Find Qualified Patients for Your Clinical Trials
Streamline patient recruitment and match participants to your specific research criteria with Quri.AI’s advanced AI-driven solutions. Save time, reduce costs, and enhance the success of your clinical trials.

Simplified SOPs Can Reduce FDA Citations and Enhance Compliance Culture
Failure to fully adhere to Standard Operating Procedures (SOPs) is a leading cause of FDA warning letters, with over half of the citations issued annually addressing this concern.

Creating a Supportive Workplace: Recognizing and Addressing Mental Health
Fostering well-being in the workplace involves raising awareness and encouraging self-care. Implementing strategies to prevent burnout and support mental health is essential for a positive, inclusive environment..

AI in Clinical Trials: Transforming Research and Regulation
Exploring AI's Role in Streamlining Clinical Trials, Addressing Regulatory Complexity, and Balancing Ethical Considerations

FDAQRC's Retreat and Reconnect
Our team experienced the wonders of Costa Rica while strengthening team bonds and making memories at the 2024 Face-2-Face annual meeting.

Pharmacovigilance: Insights, Challenges, and Future Horizons
Navigating drug safety with a focus on insights, challenges, and digital innovations for a safer future.

The Future Looks Bright
Unveiling a year of achievements, growth, and industry connections - FDAQRC sets the stage for a thriving future in 2024.

FDAQRC Named a 2024 Aggie 100 Honoree
FDAQRC is among the top 100 companies globally selected for the 19th annual Aggie 100 which honors the fastest-growing companies owned or led by Texas A&M Alum.

Celebrating 14 Years of Excellence
FDAQRC recaps a year of achievements as we celebrate 14 years in business as of October 2023!

Mastering the Difference: Monitoring and Auditing
Pharmaceutical research sponsors must make sure they follow both Monitoring and Auditing rules during their studies - so what's the difference?


Understanding the Distinctions: QA vs. QC
While these terms are sometimes used interchangeably, it is important to understand their distinctions and the specific protocols associated with each area.

We Invest In Our Employees
At FDAQRC, we understand that the best business practices must start with our employees.

National Volunteer Month
During the month of April, FDAQRC encouraged employees to donate or volunteer. Read about the impact our team made!

Applying Lean Methodology Practically
Lean Methodology is a way of optimizing the people, resources, effort, and energy of your organization toward creating value for the customer.

How to Generate Consistent Work as A Consultant
Generating consisting work seems to be a common struggle our network is seeing. Here are a few tips to set yourself up for success as a consultant and increase your number of projects.

Strategies for Combating Education Bias in Regulatory Workspaces
This downloadable poster elaborates on the problems surrounding education bias in the biopharmaceutical industry.

Explore the Pathway to Consultancy
This downloadable poster presents the clear steps of how to become a consultant while evaluating if consultancy is the best fit for someone’s experience and career goals.
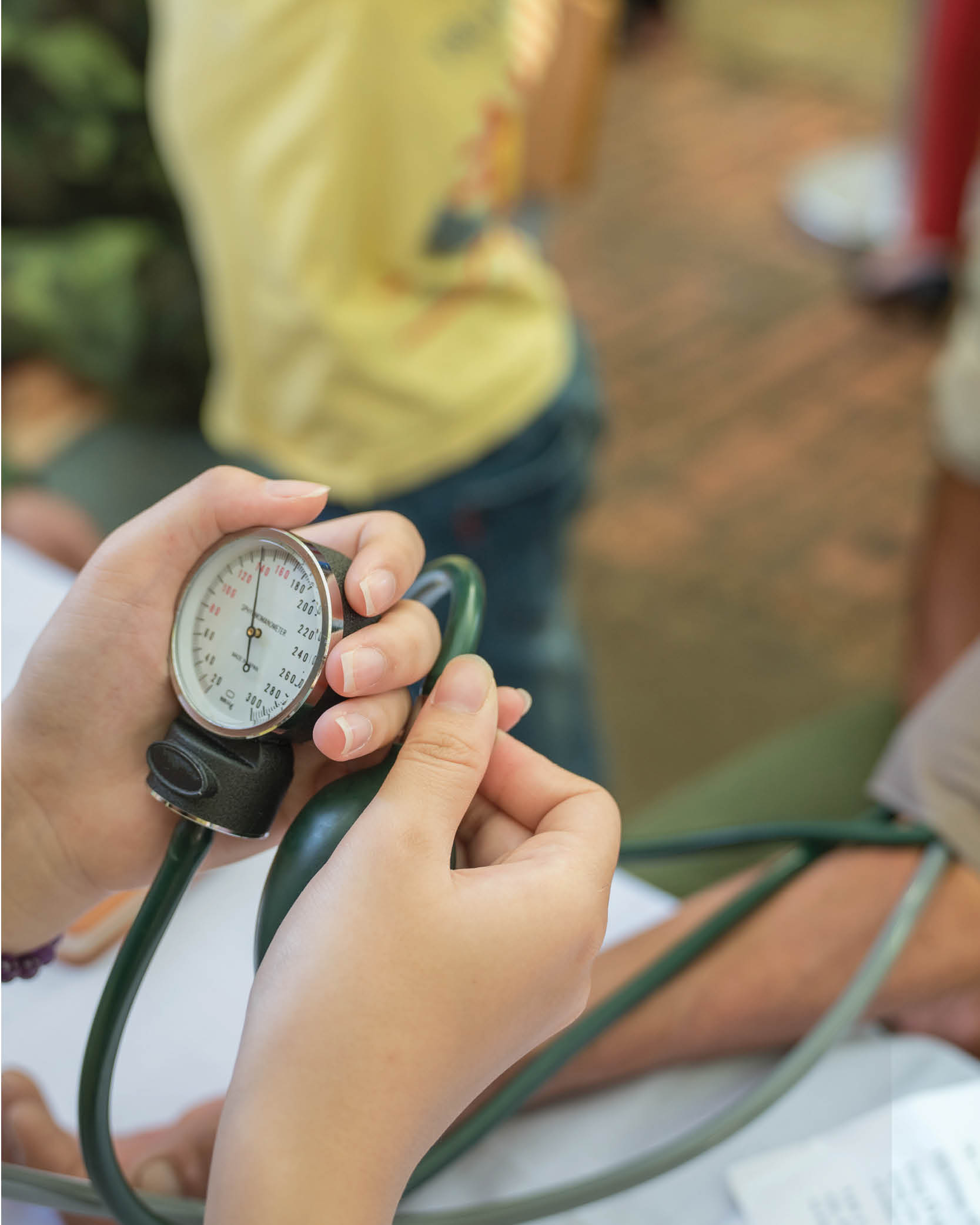
Importance of Equity in Healthcare
In honor of Black History Month we explore the need for equity in healthcare. The foundational goal is to ensure that everyone has fair access to healthcare and the right to be the healthiest version of themselves.

Transitioning From The FDA To Industry
Transitioning from the FDA into the industry can be an overwhelming experience. Here are some tips to keep in mind when entering a new role outside of the FDA.

Remediation & Continuous Improvement
Jim Darnell, Director of Remediation, presents the importance of Remediation & the Continuous Improvement process.

Happy Founder's Day, FDAQRC!
On October 12, 2022, FDAQRC will be celebrating 13 years of business. We thank our internal team, our clients, and our consultants, for our continued success!

F2F 2022: Folly Beach, South Carolina
Each year the FDAQRC team meets up for a Face-2-Face meeting. This year, our team meet up in Folly Beach, SC to look back, reflect, and plan for the future!

Avoid Common Mistakes When Submitting Your Expenses in BigTime
At FDAQRC, our consultants are a vital part of our business model. We pride ourselves on ensuring our consultants have the tools they need to succeed.

FDAQRC Experiments with a Four-Day Work Week
The new schedule was meet with an overwhelmingly positive response.

Taking Preventative Action on Regulatory Compliance Processes
FDAQRC can identify trouble areas in your compliance processes and design a plan to correct it - leaving you prepared and confident for an FDA inspection.

The War For Talent Continues
With no end in sight, FDAQRCandidates was created to help both employers and employees in this super competitive market

A Road Map To Improve Quality Culture
First presented at SQA 2022 FDAQRC’s Project Manager, Amanda White examines how poor-quality culture can take a toll on your organization.

No Regrets with Vendor Qualifications
What's required, how to begin, and how to maintain vendor qualifications.

For Cause or Not For Cause
The best way to ensure that your firm is inspection ready at all times is to be proactive in preventing “for-cause” inspections and make the reactive approach the “not to be."

A Day in the Life of a FDAQRC Auditor
From a flexible work schedule to health insurance, FDAQRC values each and every employee, but the one we’re most proud of is the fact we allow our Auditors to do what they do best, audit.

The Growing Trend Of
Quality & Regulatory Affairs
Quality & Regulatory Affairs
The advantages of Outsourcing vs. Developing In-House Capabilities

Quantifying the Quality Professional’s Experience
Try not to rely on the quantity of audits an auditor has performed, it goes against the concept of quality over quantity.


Pharma/Med Device: 2020 Year in Review
In arguably the most challenging period in modern history, the pharmaceutical and medical device industries carried on in the face of disruption to personnel, supply chains, travel, and many more critical business areas due to the Covid-19 pandemic.

Running Your
Business as
a Consultant
Business as
a Consultant
Tips, tricks, and best practices to make the most of your business.

Is Your QMS Ready
For ISO 13485:2016?
Review the five commonly asked questions from the industry regarding the transition to 13485:2016.


